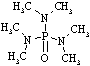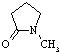| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|
| Acetic acid |
CH3COOH
60.05 |
117.9 | +16.6 | 1.0497 | miscible | miscible |
6.19 | 1.5 | 1.124 | 26.9 |
| Acetic anhydride |
(CH3CO)2O
102.09 | 140 | -73.1 |
1.075 | reacts | reacts | ? | ? | 0.9 | ? |
| Acetone |
(CH3)2C=O
58.08 |
56.3 | -94.7 |
0.7850 | miscible | miscible | 20.7 | 2.7 |
0.3040 | 22.68 |
| Acetonitrile |
CH3CN
41.05 | 81.6 |
-43.8 |
0.7768 | miscible | miscible | 37.5 | 3.44 |
0.3409 | 28.45 |
| Ammonia | NH3
17.03 | -33.4 | -77.7
|
0.6028 | 51.7 | ? | 16.9 | 1.4 | 0.135 | 19.8 |
| Aniline |

93.13 | 184.4 | -6 |
1.0175 | 3.6 | ? | 6.8 | 1.55 | 3.77 | 42.79 |
| Benzene |
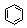
78.11 | 80.1 | +5.5 |
0.8737 | 0.18 | ? | 2.3 | 0 | 0.6028 | 28.2 |
| Benzyl alcohol |
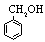
108.14 |
205.2 | -15.3 | 1.042 | 4 | ? | 13.1 | 1.7 |
? | ? |
| Bromine | Br2
159.82 | 59.5 | -7.25 |
3.1017 | 3.5 | ? | 3.2 | 0 | 0.95 | 40 |
| Bromoform | CHBr3
252.25 | 149.6 | +8.05 |
2.8776 | 0.3 | ? | 4.3 | 1.3 | 1.89 | 31.7 |
n-Butanol,
Butyl alcohol |
CH3(CH2)3OH
74.12 |
117.7 | -88.6 | 0.8057 | 8.3 | 19.7 | 17.5 | 1.66 |
2.593 | 24.3 |
tert-Butanol,
tert-Butyl alcohol |
(CH3)3C-OH
74.12 | 82.4 | +25.8 |
0.7812 | miscible | miscible | 12.4 | 1.7 | 3.35 | 19.2 |
| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|
| Carbon disulfide | CS2
76.14 |
46.26 | -111.9 | 1.2566 | 0.29 | ? | 2.6 | 0 | 0.365 | 31.5 |
| Carbon tetrachloride | CCl4
153.82 |
76.8 | -22.9 | 1.5842 | 0.08 | 0.01 | 2.2 | 0 | 0.901 | 26.2 |
| Chloroform | CHCl3
119.38 | 61.2 |
-63.5 | 1.4799 | 0.8 | 0.07 | 4.8 | 1.01 | 0.54 | 26.6 |
| Cyclohexane |

84.16 |
80.7 | +6.5 | 0.7739 |
< 0.1 | < 0.1 | 2 | 0 | 0.898 | 24.4 |
| Cyclohexanone |
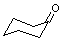
98.15 |
155.7 | -37.9 |
0.9421 | 2.4 | ? | 15 | 2.9 | 1.998 | 34.0 |
| 1,2-Dichlorobenzene |
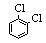
147.00 |
180.5 | -17 | 1.31 | < 0.1 |
< 0.1 | 9.8 | 2.27 | 1.32 | ? |
Dichloroethane,
Ethylene chloride | ClCH2CH2Cl
98.96 |
83.5 | -35.7 | 1.25 | 0.87 | ? | 10.66 | 1.75 | 0.83 | ? |
Dichloromethane,
Methylene chloride |
CH2Cl2
84.93 |
39.8 | -95.1 | 1.3168 |
1.3 | 0.2 | 8.9 | 1.6 | 0.423 | 27.2 |
| Diethyl ether | (CH3CH2)2O
74.12 |
34.6 | -116.3 | 0.7078 | 7.5 | 1.3 | 4.34 | 1.25 | 0.224 | 16.5 |
| Diglyme |
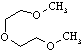
134.18 |
162 | -64 | 0.937 | miscible |
miscible | 7 | ? | 1.88 | ? |
| Diisopropyl ether |
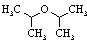
102.18 |
68.3 | -85.4 | 0.724 | 0.2 | ? | 3.8 | 1.3 | 0.3 | ? |
Diisopropyl-
-ethylamine |
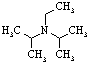
129.95 |
127 | below -50 | 0.742 | ? |
? | ? | ? | ? | ? |
| 1,4-Dioxane |

88.11 |
101.3 | +11.8 | 1.0269 |
miscible | miscible | 2.2 | 0.4 | 1.255 | 33 |
Dimethylformamide
DMF |
(CH3)2NCHO
73.10 | 153.0 | -60.4 | 0.9445 |
miscible | miscible | 37 | 3.8 | 0.796 | 36.3 |
| Dimethyl sulfate | (CH3)2SO4
126.13 |
188
decomposes | -31.8 | 1.3322 | 2.8
reacts | ? |
? | ? | ? | ? |
Dimethyl sulfoxide
DMSO |
(CH3)2S=O
78.13 | 189.0 | +18.5 | 1.0958 |
miscible | miscible | 46.7 | 3.9 | 1.996 | 43 |
| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|
Ethanol,
Ethyl alcohol |
CH3CH2OH
46.07 | 78.3 | -114.1 | 0.7851 |
miscible | miscible | 24.55 | 1.7 | 1.078 | 22 |
| Ethyl acetate | CH3COOCH2CH3
88.10 | 77.1 |
-83.9 | 0.8945 | 8.3 | 3.3 | 6.02 |
1.78 | 0.426 | 23.2 |
| Ethylene glycol |
HOCH2CH2OH
62.07 | 197.3 | -12.3 | 1.1097 |
miscible | miscible | 37.7 | 2.2 | 16.13 | 47 |
| Ethyl methyl ketone |
CH3CH2COCH3
72.11 |
79.6 | -86.7 | 0.7966 | 29.4 | 12 |
18.5 | 2.7 | 0.4 | ? |
| Formamide | HCONH2
45.04 |
210.5 | +2.5 | 1.1292 | miscible | miscible |
109.5 | 3.4 | 3.302 | 59.1 |
| Formic acid |
HCOOH
46.03 |
100.7 | +8.3 | 1.2131 | miscible | miscible |
58.5 | 1.4 | 1.8 | ? |
| Glycerol |
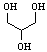
92.09 |
290.0 | +18.18 | 1.2582 | miscible |
miscible | 42.5 | ? | 945.0 | 62.5 |
| Heavy Water | D2O
20.03 | 101.42 | +3.81 |
1.11 | miscible | miscible | 78.08 | ? | 1.103 | 71.92 |
Helium
liquefied | He
4.0026 |
-268.93 | - | 0.1433 | - | ? | 1.06 | 0 |
0.0385 | 0.256 |
Hexamethyl-
phosphoramide |
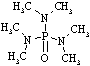
179.20 |
233 | +7.2 | 1.030 | miscible | miscible |
43.3 | 5.37 | 3.34 | ? |
| Hexane |
CH3(CH2)4CH3
86.18 | 68.7 | -95.3 |
0.6548 | < 0.1 | < 0.1 | 1.88 | ? | 0.2923 | 17.9 |
| Hydrazine | H2N-N2H
32.05 |
113.5 | +1.6 | 1.0040 | miscible | miscible |
51.7 | 1.9 | 0.905 | 66.7 |
Hydrogen azide,
Hydrazoic acid |
HN3
43.03 | 37 | -80 |
1.12 | miscible | miscible | ? | 3.0 | ? | ? |
Hydrogen cyanide,
Hydrocyanic acid |
HCN
27.03 | 25.6 | -13.4 |
0.735 | miscible | miscible | 116 | 2.88 | ? | ? |
Hydrogen fluoride,
Hydrofluoric acid |
HF
20.01 | 19.5 | -83.5 |
0.960 | miscible |
miscible | ? | 1.91 | ? | ? |
| Hydrogen peroxide | H2O2 |
150.2 | -0.4 | 1.4425 | miscible | miscible |
89(?) | 2.01 | 1.14 | 79.7 |
| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|
Iodomethane
Methyl iodide | CH3I
141.94 |
42.5 | -66.5 | 2.28 | 1.4 | ? |
? | 1.6 | 0.5 | ? |
| Isobutanol |
(CH3)2CHCH2OH
74.12 |
107.7 | -108 | 0.798 | 10 | 16 |
17.9 | 1.79 | 6.68 | ? |
Isobutyl methyl
ketone |
(CH3)2CHCH2COCH3
100.16 |
116.5 | -84.0 | 0.80 | 1.9 | 1.8 |
13.1 | ? | 0.59 | ? |
Isopropanol,
2-Propanol | (CH3)2CHOH
60.10 |
82.3 | -88 | 0.7810 | miscible | miscible |
19.9 | 1.66 | 2.073 | 18.3 |
| Mercury | Hg
200.59 | 356.9 | -38.83 | 13.534 |
3.6 · 10-7 | ? | - | 0 | 1.52 | 473.5 |
| Methanol |
CH3OH
32.04 |
64.7 | -97.7 | 0.7866 | miscible | miscible |
32.6 | 1.6 | 0.5445 | 22.1 |
| N-Methylformamide |
HCONHCH3
59.07 |
182 | -4 | 0.9988 | miscible | miscible |
182.4 | 3.8 | 1.65 | 38.07 |
| N-Methypyrrolidone |
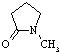
99.13 |
202 | -24 | 1.033 | miscible | miscible |
33 | 4.1 | 1.8 | ? |
Nitric acid
fuming |
HNO3
63.01 |
83.9 | -41.6 | 1.5129 | miscible | miscible |
50 | 2.2 | 0.749 | 41.2 |
| Nitrobenzene | 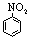
123.11 |
210.8 | +5.8 | 1.1987 | 0.19 | ? | 34.8 | 4.3 |
1.823 | 42.8 |
Nitrogen
liquefied | N2
28.01 |
-195.8 | -210.0 | 0.8409 | - | ? | 1.45 | 0 |
0.2039 | 10.53 |
| Nitromethane | CH3NO2
61.04 |
101.2 | -28.5 | 1.1312 | 9.5 | 1.9 | 35.87 | 3.1 |
0.627 | 36.2 |
| Oxalyl chloride |
(COCl)2
126.93 |
63 | -12 | 1.48 |
reacts
violently | reacts
violently |
? | ? | ? | ? |
| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|
| Phosphorous trichloride | PCl3
137.33 | 76 | -94 | 1.55 | reacts
violently | reacts
violently | ? | 1.16 | ? | ? |
| Phosphoryl chloride | POCl3
153.33 | 108 | +1.15 | 1.648 | reacts
violently | reacts
violently | 13.9 | 2.4 | 1.15 | 31 |
| Propylene carbonate | 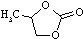
102.09 |
241.7 | -50.0 | 1.20 | 24 | ? | 69.0 |
? | ? | ? |
| Pyridine | 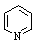
79.10 |
115.3 | -41.6 | 0.9779 | miscible | miscible |
12.4 | 2.2 | 0.8826 | 36.5 |
| Sulfolane | 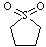
120.17 |
287.3 | +28.4 | 1.26 | miscible | miscible | 43.3 |
4.8 | 10.1 | ? |
| Sulfur dioxide | SO2
64.06 | -10.02 | -75.5 |
1.3719
| 10.5 | ? | 12 | 1.6 | 0.35 | 21.6 |
| Sulfuric acid |
H2SO4
98.08 |
274 | +10.35 | 1.8305 | miscible | miscible |
101 | 2.7 | 25.54 | ? |
| Sulfuryl chloride | SO2Cl2
134.97 |
69.1 | -54.1 | 1.67 | reacts
violently | reacts
violently |
? | 1.8 | 0.92 | ? |
Tetrahydrofurane,
THF |

72.11 | 66.0 |
-108.5 | 0.8844 | miscible | miscible |
7.58 | 1.6 | 0.461 | 26.4 |
| Thionyl chloride | SOCl2
118.97 |
78.8 | -105 | 1.64 | reacts
violently | reacts
violently |
? | 1.4 | 0.6 | ? |
| Toluene |

92.14 | 110.6 | -95.0 |
0.8623 | 0.052 | < 0.1 | 2.38 | 0.36 |
0.552 | 27.8 |
| Triethylamine | (CH3CH2)3N
101.19 | 89.5 | -114.7 |
0.7235 | 13.3 | ? | 2.44 | 0.82 | 0.341 | 20.1 |
Trimethylsilyl
chloride |
(CH3)3SiCl
108.64 |
57 | -58 | 0.856 | reacts
violently | reacts
violently |
? | ? | 0.4 | ? |
| Water | H2O
18.02 | 100.0 | 0.0 |
0.997 | - | - | 78.39 | 1.84 | 0.8905 | 71.98 |
| Name | Structure and
molecular weight |
Boiling
point
°C |
Melting
point
°C |
Density
g/ml |
Solubility in
100g of water |
Solubility of
water in 100g
of solvent |
e - Dielectric
constant |
Dipole
moment |
Viscosity
10-3 Pa · s |
Surface
tension
10-3 J/m2 |
|---|